Draw all stereoisomers of 2-bromo-4-methylpentane – Embark on a journey into the fascinating world of stereoisomers as we delve into the complexities of 2-bromo-4-methylpentane. This comprehensive guide unravels the intricate relationship between structure, chirality, and molecular properties, providing a deeper understanding of this captivating realm of chemistry.
Our exploration begins with an in-depth analysis of structural isomers, unveiling the diverse molecular arrangements that give rise to compounds with identical molecular formulas. We then delve into the concept of stereoisomers, examining the subtle differences in spatial orientations that distinguish enantiomers and diastereomers.
Structural Isomers
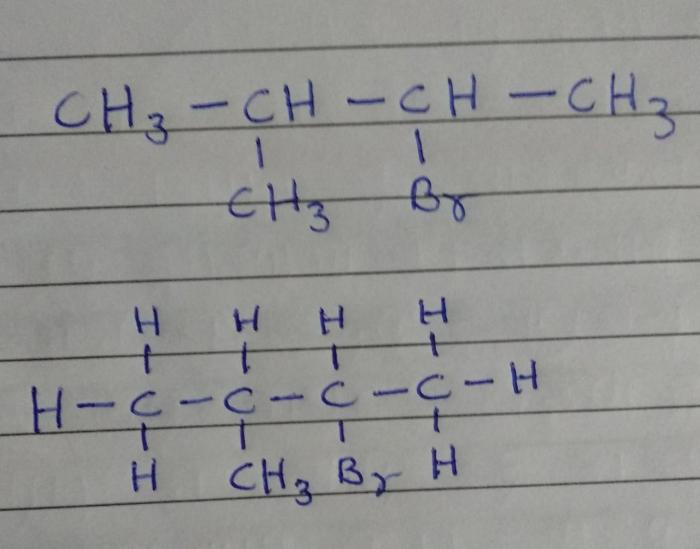
Structural isomers are compounds that have the same molecular formula but different structural formulas. 2-bromo-4-methylpentane has two structural isomers:
- 2-bromo-4-methylpentane
- 4-bromo-2-methylpentane
Stereochemistry: Draw All Stereoisomers Of 2-bromo-4-methylpentane
Stereoisomers are compounds that have the same molecular formula and structural formula, but differ in the spatial arrangement of their atoms. 2-bromo-4-methylpentane has one stereocenter, which is the carbon atom that is bonded to the bromine atom. This carbon atom has four different groups attached to it, so there are two possible spatial arrangements of these groups.
The two stereoisomers of 2-bromo-4-methylpentane are:
- (2 R,4 S)-2-bromo-4-methylpentane
- (2 S,4 R)-2-bromo-4-methylpentane
Enantiomers
Enantiomers are stereoisomers that are mirror images of each other. The two stereoisomers of 2-bromo-4-methylpentane are enantiomers.
Enantiomers have the same physical properties, but they differ in their chemical properties. For example, enantiomers react differently with chiral reagents.
Diastereomers
Diastereomers are stereoisomers that are not enantiomers. The two stereoisomers of 2-bromo-4-methylpentane are diastereomers.
Diastereomers have different physical and chemical properties. For example, diastereomers have different melting points and boiling points.
Meso Compounds

A meso compound is a compound that has a plane of symmetry. 2-bromo-4-methylpentane does not have a plane of symmetry, so it is not a meso compound.
Meso compounds have the same physical and chemical properties as their enantiomers. However, meso compounds are not optically active.
User Queries
What is the IUPAC name for 2-bromo-4-methylpentane?
2-Bromo-4-methylpentane
How many stereoisomers does 2-bromo-4-methylpentane have?
4
Are the enantiomers of 2-bromo-4-methylpentane optically active?
Yes
